Abstract
Introduction: There is no effective standard of care for older AML pts who are unfit for induction. We explored the combination of the BCL-2 inhibitor venetoclax (ven) with azacitidine (aza) for this population in a multi center phase 1b study (NCT02203773), and report clinical results from our center, as well as mechanistic insights regarding the activity of this regimen derived from correlative studies performed with our pt samples.
Methods: The dose escalation/expansion trial combined aza or decitabine, at the standard dose and schedule, with ven daily in older AML pts unfit for induction. Our institution enrolled into the aza arm. Pts were eligible if ≥65 and with adequate organ function. Response assessments employed International Working Group (IWG) criteria; if a morphologic remission was achieved without count recovery, delay of the subsequent cycle by up to 14 days was permitted, with growth factors per institutional guidelines, and best response was upgraded if counts improved in this timeframe. Minimal residual disease (MRD) testing was performed by digital droplet PCR (ddPCR) with a sensitivity of 0.01%. Mass cytometry was performed using CyTOF.
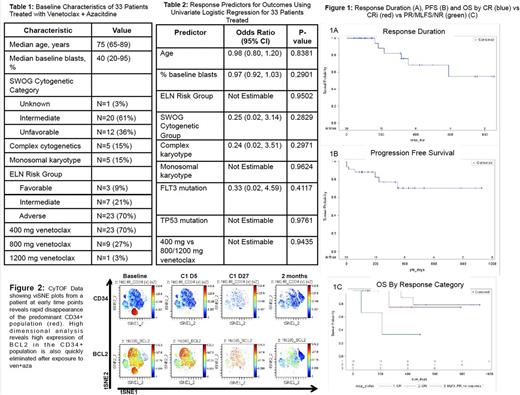
Results: 33 pts enrolled and received at least 1 day of therapy. 23 received 400 mg venetoclax, 9 received 800 and 1 received 1200 (Table 1). 2 elected to come off study after <7 days; both died of AML. Median follow up time using reverse Kaplan-Meier was 351 days (95% CI 146, 468). The overall response rate (ORR), defined as complete response (CR) + CR with incomplete count recovery (CRi) + partial response (PR) + morphologic leukemia free state (MLFS), was 30/33 (91%); 19 (58%) had a CR, 9 (27%) had a CRi, 1 had a PR and 1 had a MLFS. Only 1 pt who completed >1 week of therapy did not respond (resistant disease). No variables tested predicted outcomes in the 31 pts who had >1 week of therapy (Table 2); 5/5 with monosomal karyotype, 5/5 with FLT3 and 2/2 with TP 53 responded. 3 proceeded to transplant; none have relapsed after 14, 8 and 4 months. 4/33 (12%) relapsed; only 1 relapse occurred in a patient who continued on therapy, after 13 months. 3/4 relapsed after coming off study for a hip fracture (N=1), personal decision (N=1) and development of mastocytosis (N=1). The median response duration has not occurred; mean is 484 days (std error 44). Median progression free survival (PFS) has not occurred; mean is 294 days. Median overall survival (OS) has not occurred; mean is 376 days (Figure 1).
We used ddPCR to measure MRD in all pts with amenable mutations (N=18). Of these, 14 have completed ≥1 cycle with ≥3 months of follow-up, and were analyzed for MRD. 3 achieved MRD negativity; 1 who remains on therapy (cycle 16, 453 days), and surprisingly, 2 who discontinued therapy, for personal reasons and cytopenias, after 1 and 6 cycles. They remain in MRD negative CR and CRi after 24 and 16 months. Another who achieved a CR and discontinued therapy after 3 cycles for personal reasons has also had no further therapy for 25 months and continues to be in CR with borderline MRD detectability (0.024%).
Detailed temporal analysis of 2 pts who achieved a CR and CRi using CyTOF and single cell RNA-seq methods showed rapid and extensive targeting of primitive AML cell types (Figure 2, not all data shown). This data indicates effective targeting of the AML stem cell population. Ongoing functional studies using xenograft models will corroborate direct targeting of AML stem cells.
Conclusion: Ven with aza is a highly active regimen for untreated elderly AML pts who are not candidates for induction chemotherapy; all but 1 pt who stayed on study for >1 week achieved an IWG response, primarily CR/CRi. There were no serious adverse events related to ven and very few significant related adverse events. Responses were durable with few relapses, particularly for pts who remained on treatment; 3 pts who elected to stop all therapy have had no recurrence with considerable follow up. MRD detection with ddPCR reveals the depth of response possible with aza+ven; several pts in long term follow up have achieved MRD negative remissions, including 2 who discontinued all therapy after 6 months or less with no subsequent treatment for 24 and 16 months. Median PFS and OS were not reached with median follow up time of nearly 1 year. The universality, depth and durability of responses, coupled with the CyTOF and single cell RNA-seq data, suggest this regimen effectively targets the leukemia stem cell population.
Pollyea: Agios, Pfizer: Research Funding; Takeda, Ariad, Alexion, Celgene, Pfizer, Pharmacyclics, Gilead, Jazz, Servier, Curis: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal